Packaging Symbol Glossary
SYMBOL
DESCRIPTION
ISO 15223-1
REF. NO.
REF. NO.

Manufacturer
The date of manufacture, as well as the name and address of the manufacturer, can be combined in one symbol.
Example: Name and Address
YYY-MM-DD
Name and Address
YYY-MM-DD
The date of manufacture, as well as the name and address of the manufacturer, can be combined in one symbol.
Example:
 Name and Address
YYY-MM-DD
Name and Address
YYY-MM-DD
5.1.1

Country of manufacture
5.1.11

Authorized representative in the European community/ European Union
(To be replaced by EU REP symbol, see below.)
(To be replaced by EU REP symbol, see below.)
5.1.2

Authorized representative in the European Union
(Replaces EC REP symbol when expired.)
(Replaces EC REP symbol when expired.)
5.1.2

Authorized representative in Switzerland
5.1.2

Date of manufacture
5.1.3

Use-by date
5.1.4

Batch Code
5.1.5

Catalogue number
5.1.6

Importer
5.1.8
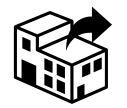
Distributor
5.1.9

Sterilized using ethylene oxide
5.2.3

Sterilized using irradiation
5.2.4

Non-sterile
5.2.7

Do not use if package is damaged and consult instructions for
use
For products which do not have instructions for use, the instruction to consult them does not apply.
For products which do not have instructions for use, the instruction to consult them does not apply.
5.2.8
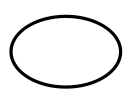
Single sterile barrier system
5.2.11
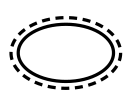
Single sterile barrier system with protective packaging
outside
5.2.14
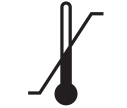
Temperature limitation
5.3.7
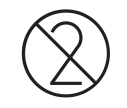
Do not re-use
5.4.2

Consult instructions for use
5.4.3

Caution
5.4.4
SYMBOL
DESCRIPTION
ISO 15223-1
REF. NO.
REF. NO.

In vitro diagnostic medical device
5.5.1

Contains sufficient for <n> tests
5.5.5

Medical Device
5.7.7

Unique device Identifier
5.7.10

Authorized representative in the United Kingdom
*

CE marking of conformity
Indicates a product conforms with one or more directives or regulations issued by the European Parliament and the Council of the European Union.
Indicates a product conforms with one or more directives or regulations issued by the European Parliament and the Council of the European Union.
N/A

CE marking of conformity
Indicates a product conforms with one or more directives or regulations issued by the European Parliament and the Council of the European Union. Devices that have been approved by a Notified Body and issued an EC Certificate include the 4-digit identifier for the Notified Body.
Indicates a product conforms with one or more directives or regulations issued by the European Parliament and the Council of the European Union. Devices that have been approved by a Notified Body and issued an EC Certificate include the 4-digit identifier for the Notified Body.
N/A

For prescription use only in the United States
N/A

Not made with natural rubber latex
N/A

Properly dispose of used product as potential biohazardous
material and waste
Dispose of in accordance with local, regional, national, international regulations as specified.
Dispose of in accordance with local, regional, national, international regulations as specified.
N/A

Quantity of devices
N/A

Made in USA
N/A
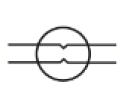
Breakpoint
N/A
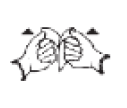
Pull apart wrapper
N/A

Pull apart wrapper
N/A

Pull apart wrapper
N/A
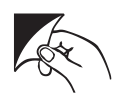
Pull apart wrapper
N/A
N/A = No normative standards reference
* = www.gov.uk/guidance/using-the-ukca-marking#when-to-use-the-ukca-marking


